Gary Kearns, César Fernández-De-Las-Peñas, Jean-Michel Brismée, Josué Gan & Jacqueline Doidge (2019): New perspectives on dry needling following a medical model: are we screening our patients sufficiently?, Journal of Manual & Manipulative Therapy, DOI: 10.1080/10669817.2019.1567011
To link to this article: https://doi.org/10.1080/10669817.2019.1567011
a Physical Therapy (DPT) Program, Department of Rehabilitation Sciences, School of Health Professions, Texas Tech University Health Sciences Center, Lubbock, TX, USA; b Department of Physical Therapy, Occupational Therapy, Rehabilitation and Physical Medicine, Universidad Rey Juan Carlos, Alcorcón, Madrid, Spain; c Cátedra de Investigación y Docencia en Fisioterapia: Terapia Manual y Punción Seca, Universidad Rey Juan Carlos, Alcorcón, Madrid, Spain; d Departamento de Fisioterapia, Facultad de Ciencias de la Salud, Universidad Rey Juan Carlos, Alcorcón, Madrid, SPAIN; e Center for Rehabilitation Research & Doctor of Science (ScD) Program in Physical, Department of Rehabilitation Sciences, School of Health Professions, Texas Tech University Health Sciences Center, Lubbock, TX, USA; f Institute of Physiotherapy, School of Health Professions, Zurich University of Applied Sciences ZHAW, Winterthur, Switzerland; g Physiotherapie Bösch, Bern, Switzerland; h Optimal Dry Needling Solutions, Tuscon, AZ, USA
ABSTRACT Myofascial trigger points are not an isolated neuromusculoskeletal phenomenon and have been implicated in systemic, visceral, and metabolic pathology, as a side effect of some medications and in the presence of psychological risk factors. This complexity can complicate adequate screening of patients prior to choosing dry needling as a treatment intervention. Regardless of whether clinicians practice in a direct access setting, they should be cognizant of medical conditions, comorbidities, and risk factors that will influence clinical decisions for dry needling appropriateness, technique chosen, and potential adverse responses to treatment. Of primary concern are conditions that can either manifest with myalgia and/or myopathy or masquerade as a more common musculoskeletal condition. This clinical commentary reviews system-specific considerations and other common disorders that should be screened for and discusses not only whether dry needling is appropriate but comments on technique and dosage considerations when initiating dry needling.
KEYWORDS Dry needle; trigger point; safety; screening
Introduction
A myofascial trigger point (MTrP) is defined as a hyper- irritable spot in skeletal muscle that is painful on compression, stretch, overload, or contraction of the tissue, which usually responds with a referred pain that is perceived distant from the spot [1]. MTrPs are identified during palpation and are commonly treated with, but not limited to, trigger point injection, ischemic compression, myofascial release, soft tissue mobilization, and dry needling [2]. While there has been a recent focus on the safety of dry needling application [3–7] and the potential for adverse events [4–6,8], one area that seems to be missing is context. Most papers focus on proper and deep knowledge of anatomy for safe application of dry needling [4,5]. For instance, proper anatomical knowledge and clinician’s skill can avoid adverse events such as pneumothorax when needling thoracic muscles [8]. The presence of MTrPs is not pathognomonic with mechanical neuromusculoskeletal dysfunction. MTrPs have been implicated in chronic pain conditions involving sensitization, systemic autoimmune pathology, and visceral pathology, as a side effect of some medications, and in the presence of psychological risk factors [9]. This underscores the complexity that goes into adequately screening our patients prior to choosing dry needling as a treatment intervention. In 2013, The American Physical Therapy Association (APTA) [10] released a resource paper summarizing the contraindications and precautions that should be considered prior to dry needling. These include needle phobia, cognitive impairments, communication impairments, patient refusal, isolated skin lesions, systemic infections, local lymphedema, severe hyperalgesia or allodynia, allergies to metals, immunocompromised patients, pregnancy during thefirst trimester, vascular disease, and increased bleeding tendencies [10]. While screening for conditions on this list is necessary, many clinicians may view it as common sense with any invasive technique.
In 2015, The Federation of State Boards of Physical Therapy (FSBPT) [11] released competencies that physical therapists should possess to safely integrate dry needling into clinical practice. Their conclusion was that clinicians should be well rounded in all areas of patient care including subjective history, medical screening, systems review, objective examination, selection of treatment techniques in conjunction with dry needling, and continuing reassessment. Unfortunately, there is a paucity of literature, save for textbooks [9,12], that addresses more thorough medical screening of at- risk patients who may be inappropriate for dry needling or have an adverse reaction to dry needling. In fact, many of the areas recommended to screen [10,11] are either obvious or broad and lack sufficient detail to provide adequate context when integrating dry need- ling into patient care.
Regardless of whether clinicians who utilize dry needling practice in a direct access setting, they should function within the medical model context and be cognizant that patients are attending physical therapy with a potential litany of risk factors and comorbidities that will influence clinical decisions for appropriateness of dry needling, dry-needling technique chosen, and potential adverse responses to treatment. Of primary concern are medical conditions that can either manifest with myalgia and/or myopathy or present with symptoms that may be mistaken for more common musculoskeletal conditions. It merits reviewing some of the system-specific considerations and other common disorders that should be screened for and discuss not only whether dry needling is appropriate but comment on technique and dosage considerations when initiating dry needling.
Dry-needling technique and dosage considerations
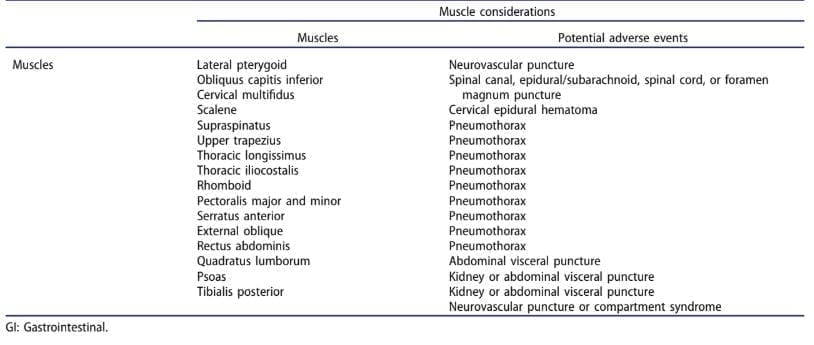
There is currently a lack of consensus on the most appropriate technique and dosage of dry needling. This may complicate clinical reasoning during medical screening to identify patients that are appropriate to receive dry needling. There are many theoretical models [2,13–17] that have influenced clinicians practicing dry needling. The fast-in-and-fast-out technique described by Hong [17] is probably the most widely practiced but the literature is controversial in relation to the use of this technique [18] and scarce in relation to dry-needling dosage [19] or comparison of different techniques [20]. Some variables that should be considered for a single treatment session include the number of needles used, the number of muscles treated, the speed and depth of pistoning [21], and the type of needle manipulation. While there is little in the literature on this topic, clinical experience suggests that some techniques and dosages such as multiple needle manipulations and entries are more aggressive than others such as minimum needle manipulations and few entries, which may be better suited in specific patient presentations. In addition, the targeted region or specific muscle should be also included in this equation to determine the benefit-risk ratio in each patient (Table 1).
The following sections have been organized in alphabetical order of potential comorbidities and medications interactions to be considered while screening patients prior to dry needling treatment application. While most statements are referenced using the literature, some are derived from clinical judgment due to the lack of literature on selected topics. 2 G. KEARNS ET AL.
Biopsychosocial
Recently, much attention has been devoted to risk factors such as anxiety, stress, and fear avoidance in the presence of chronic pain states. Adequate screening for such risk factors can be gleaned from a thorough subjective history or using standardized outcome measures such as the Fear Avoidance Beliefs Questionnaire [22], The McGill Pain Questionnaire [23], Pain Catastrophizing Scale [24], and Central Sensitization Inventory [25]. While the presence of these risk factors should not preclude dry needling as an intervention, the clinician should consider how these risk factors could impact safely applying dry needling or exacerbating the risk factors. With patients in chronic pain states, proper screening for the presence of allodynia, hyperesthesia or hyperpathia is essential to determine whether the patient is an appropriate candidate for manual therapy [26], including dry needling. More aggressive dry- needling techniques or overdosage may be perceived as excessively noxious to the patient and may result in a poor outcome or pain exacerbation. This emphasizes the importance when selecting dry needling, to choose appropriate dosing to avoid overtreatment and gentler techniques to minimize the risk of symptom exacerbation.
Cancer
There is growing evidence for the presence of MTrPs and their potential role in oncologic pain [27–29]. While there is support for conservative treatments targeting MTrPs [29], including ultrasound-guided injections [28] and dry needling [27], clinicians should perform a thorough screening of medical treatments including radiation and chemotherapy. Some of the pertinent side effects of chemotherapy and radiation include immunosuppression, anemia, infection, increased bleeding, atrophy and weakening of soft tissues, and delayed wound healing [30]. Patients receiving oncology treatment should be viewed with caution before initiating dry needling as there may be an increased risk of infection, hematoma, bruising, delayed healing, or a poor response to more aggressive techniques. Should dry needling be chosen, a trial of a gentler technique [21] such as fewer number of needles with minimal needle manipulation [27] would be warranted.
Table 1

Cardiovascular and hematologic disease
Bleeding and bruising are two of the more common dry needling side effects [7]. Being aware of hematologic disorders such as thrombocytopenia, hemophilia, and other factor deficiency clotting disorders [12,31] is imperative to minimize the risk of hematoma and bruising. Should dry needling be chosen, a trial of a gentler technique [21] such as fewer number of needles or minimal needle manipulation would be warranted. Clinicians should ensure adequate hemostasis in this at-risk population. For instance, two case reports describe acute cervical epidural hematoma after dry needling of the cervical multifidus [32,33]. Although not directly related to the presence of vascular disease in these particular patients, dry needling of a patient with a comorbid vascular condition could further complicate a more serious adverse event such as epidural hematoma. Additionally, patients with blood disorders such as sickle cell disease, leukocytosis, leukopenia, and thrombocytopenia often have a potentially higher risk of infection [31] due to possible concomitant immunosuppression. Cardiovascular and hematologic risk factors should not be viewed as a contraindication to dry needling, rather, regarded with caution to minimize the risk of bleeding, bruising, or infection. Application of dry needling with these populations may be considered following adequate screening of risk factors and education of the patient regarding potential adverse events.
Gastrointestinal/Hepatic/biliary
While most gastrointestinal (GI) conditions do not manifest with musculoskeletal complaints, it bears mentioning both ulcerative colitis and CrohnLs disease. Collectively inflammatory bowel disease, one hallmark of both conditions, is migratory arthralgias that can often precede the emergence of GI symptoms such as nausea, diarrhea, constipation, or other constitutional signs and symptoms [31]. Although arthralgia is commonly present, myalgia [34,35] may also be one of the first manifesting symptoms. This underscores the importance of screening for prior history of ulcerative colitis or CrohnLs disease. Close reassessment of palpation findings posttreatment and patient response to treatment must be viewed in conjunction with continual monitoring and reevaluation of the emergence of any GI symptoms.
Patients with gallbladder disease, especially in early presenting cases, have been reported to display associated pain in right-sided spinal muscles supplied by the anterior horn of segments innervating the affected portion of the gallbladder [31]. Without adequately screening for associated constitutional symptoms, worsening of symptoms with intake of food, prior history of GI pathology, or thorough abdominal screen including assessing for the presence of a positive MurphyLs sign [31], a scenario is presented where provocative palpation may lead to choosing dry needling as an intervention. It is important to consider that GI conditions may activate the viscero-somatic reflex producing MTrPs in muscles such as the rectus abdominis or thoracic paraspinals that are supplied by common spinal segments of the diseased organ. Without placing palpation findings in the overall context of the patientLs presentation, dry needling may be applied to a patient that is inappropriate or may require referral to a physician. Clinicians should be prudent in screening GI conditions when considering needling of the trunk musculature [6].
Inflammatory autoimmune disorders
Inflammatory autoimmune disorders such as rheumatoid arthritis, reactive arthritis, ankylosing spondylitis, systemic lupus erythematosus, and psoriatic arthritis may be encountered in clinical settings for conservative management of (4 G. KEARNS ET AL. ) associated neuromusculoskeletal impairments causing activity limitations [30,36–39]. While clinical presentation is variable, many of the common musculoskeletal manifestations include myalgia and arthralgia [30]. One concern in this population is overly aggressive treatment during relapses or active symptomatic flares. Most relapses or flare of symptoms has been attributed to tapering off or stopping biologics [40] or poorly controlled states. While nothing has been addressed in the literature specific to physical therapy treatment causing worsening of flares while tapering off biologics, Goodman et al. [41] found an increased frequency of symptomatic flares in patients with rheumatoid arthritis following total hip and total knee arthroplasty. While inflammatory autoimmune disorders should not preclude dry needling, proper screening of the stability of the disease and medication status is merited to avoid overly aggressive dry-needling dosage and technique in unstable or active flares to prevent exacerbating symptoms. Despite support for needle-based therapies such as acupuncture in rheumatoid arthritis [42], clinicians should consider avoiding the application of dry needling during the active phase of chronic autoimmune inflammatory diseases, however may consider application during the stationary or remittent phase.
Integumentary
Aside from routine screening [31,43] for signs of active infection, open wounds, or inflammation, the tissue integrity and healing potential should be considered prior to dry needling [12]. Tissue integrity can be affected by chronic systemic conditions such as rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, and diabetes [30]. Chemotherapy, radiation treatment, and prolonged use of medications such as corticosteroids also may impact the ability to tissues to heal [30,44,45]. These considerations must be viewed with caution before proceeding with dry needling in these patients as there may be an increased incidence or severity of hematoma, bruising, or a poor response to more aggressive techniques.
Medications
Screening for potential deleterious effects of medications that increase the probability of posttreatment bleeding such as anticoagulants is common [12]. Additionally, immunosuppressive medications should be viewed with extreme caution for increased risk of infection [12]. Little attention, however, has been given to myalgia as a potential side effect of medications. Medications that may present with myalgia or myopathy include statins, glucocorticoids, immunologics, antimicrobials, antiviral therapy, and antimalarials [46–48]. Polypharma in patients presenting with pain may be viewed as a reason to choose a non-pharmacologic intervention such as dry needling for pain relief, but not adequately considering side effects of medication and over relying on provocative palpation findings, clinicians may inappropriately apply dry needling into a population that requires physician consultation. Continual reassessment of patient response to treatment is essential to ensure subjective, objective, and functional progress is occurring.
Metabolic and endocrine disorders
Diabetes is one of the more common metabolic disorders seen as a comorbidity in patients presenting to physical therapy [49,50]. Of particular concern to the clinician performing dry needling are the pathological neural and vascular complications of long-term or poorly controlled diabetes. Diabetic neuropathy is one of the more severe consequences of diabetes and can result in sensory impairment (paresthesia and numbness), complaints of burning pain, and extreme sensitivity to touch [31]. There are several considerations for choosing dry needling in patients with diabetic neuropathy. If impaired sensation is present, the patientLs ability to accurately subjectively report pain provocation during both assessment and treatment may be impaired. Additionally, impaired sensation should be viewed as a precaution to invasive techniques. Lastly, if hyperesthesia is present, clinicians should consider whether needle insertion with subsequent manipulation will be noxious to the patient, resulting in increased pain perception. Should dry needling be initiated, choosing a trial of a lighter technique [21] such as fewer number of needles or minimal needle manipulation would be warranted to gauge patient response.
Both large and small vessel disease occur at an earlier age and progress faster in patients with diabetes [31]. Particularly in the extremities, microvascular changes result in poor perfusion of the tissues, potential ischemic pain, and increase the chance of poor or delayed healing as well as a higher risk of infection or cellulitis [21,31]. With these pathologic changes, clinicians should use caution when considering dry needling, especially aggressive techniques, in diabetic patients to reduce the chance of infection or adverse responses, particularly when needling lower extremity muscles.
Other comorbidities that may be encountered in the clinical setting include endocrine disease processes. While there are more serious systemic manifestations, conditions such as hyperthyroidism, hypothyroidism, hyperparathyroidism, hypoparathyroidism, primary adrenal insufficiency, and CushingLs Syndrome may manifest with early musculoskeletal complaints of myalgia, muscle cramping, and fatigue [31]. Although dry needling is not contraindicated in these conditions, clinicians should continually screen for endocrine disease and the appearance of any additional systemic symptoms that may require physician referral.
Urogenital disease
Pain presenting in the thoracolumbar spinal region, although not common, can be attributed to musculoskeletal pathology and impairments [51–53] that include provocation of pain with palpation of muscles in this region. James Cyriax, M.D. [54] described the forbidden area in the lower thoracic and upper lumbar spine, emphasizing that many of the pain presentations in this region are from pathology outside of the musculoskeletal system and should be viewed with suspicion before proceeding with treatment. Upper urinary tract pathology, particularly early presentations of kidney infection or inflammation, can closely mimic musculoskeletal pain and present with lower thoracic costovertebral tenderness and T10-L2 skin hypersensitivity [31] before the emergence of urinary or constitutional symptoms. Clinicians must be aware and screen for prior history of kidney disease, continue to monitor the emergence of additional symptoms such as dysuria, hematuria, or change in frequency or urgency [31], and may utilize the Murphy Percussion Test over the kidneys [31,55] as a screen to cluster with other findings suggestive of kidney disease.
Similar to screening for gallbladder dysfunction, provocative palpation cannot be the sole determining finding that guides choosing dry needling as an intervention. Without placing the palpation findings into the overall context of the patientLs presenting complaints, dry needling can be misapplied to a patient requiring referral to a physician. In fact, the term chronic pelvic pain syndrome, which include conditions such as bladder pain syndrome, endometriosis pain syndrome, interstitial cystitis, prostatic pain syndrome, dysmenorrhea, and vulvodynia, is associated to the presence of MTrPs [56]. While these conditions may be responsive to needling therapies [57], they may also warrant physician consultation.
Conclusion
The APTA [10] and FSBPT [11] have provided a concise summary of pertinent screening considerations as well as skills clinicians should possess to adequately ensure patients are appropriate for dry needling. Clinicians must reason and make treatment decisions within the context of the medical model being particularly aware of screening for disease processes or conditions that can manifest with myalgia or influence how a patient will respond to dry needling. Clinicians should be strongly cautioned against relying solely on palpation findings when choosing dry needling without thorough systems screening and viewing provocative findings within the patientLs overall presentation. While many of the conditions mentioned require precautionary care and are not absolute contraindications to performing dry needling, special consideration of appropriate dosage and technique chosen may reduce the risk for an adverse response to treatment and improve clinical outcomes. Most clinical decisions regarding patient care, including dry needling, require clinical reasoning where it is essential that physical therapists use the patient history to establish and test hypotheses related to potential dry-needling adverse events. Physical therapists are responsible to recognize and weigh the risks to benefits ratio for each patient and to do whatever is reasonable to minimize risks and enhance the benefits associated with dry-needling intervention using their clinical reasoning skills.
Disclosure statement
No potential conflict of interest was reported by the authors. The primary author teaches dry needling as a guest faculty member for the North American Institute of Orthopaedic Manual Therapy. Cesar Fernandez-de-las-Penas and Josue Gan are instructors in Trigger Ponit Therapy and Dry Needling for David G. Simons Academy. Jacqueline Doidge is Co-Director and Senior Lecturer for Optimal Dry Needling Solutions.
Notes on contributors
Gary Kearns is an assistant professor in the Doctor of Physical Therapy Program at Texas Tech University Health Sciences Center. He is a fellow in the American Academy of Orthopedic Manual Physical Therapists (FAAOMPT) and his fellowship project entitled Medical Diagnosis of Cubital Tunnel Syndrome Ameliorated with Thrust Manipulation of the Elbow and Carpals was published in the Journal of Manual and Manipulative Therapy in 2012. He graduated with his Doctor of Science (ScD) in Physical Therapy through Texas Tech University Health Sciences Center in 2015. Most recently, he became a board-certified specialist in Orthopedic Physical Therapy (OCS) in 2016.
César Fernández-de-Las-Peñas is a professor of physical therapy at Universidad Rey Juan Carlos, Madrid, Spain where he is the head division of a clinical research group focusing on pain sciences. Cesar has 18 years clinical experience in private practice and pain clinic, focusing on manual therapy approaches for the management of chronic myofascial pain. He conducted his PhD in biomedical sciences in the Centre for Sensory Motor Interaction (SMI) in Aalborg University, Denmark and a second PhD in Physical Therapy at the Universidad Rey Juan Carlos. His research activities concentrate on biomedical sciences within neuroscience, specifically on pain science and assessment in chronic pain patients and healthy subjects. He has over 400 scientific publications, being leading researcher of approximately 150 of them. Most publications concentrate on neck pain, headaches, carpal tunnel syndrome, lateral epicondylalgia, and the neurophysiological effects of manual therapy. In addition, Cesar is a renowned lecturer worldwide and a distinguished guest speaker in conferences. 6 G. KEARNS ET AL.
Jean-Michel Brismée is a professor in the Doctor of Science Program of Physical Therapy and Doctor of Philosophy Program of Rehabilitation Sciences at Texas Tech University Health Sciences Center. He has authored over 100 scientific papers in refereed journals related to musculoskeletal physical therapy practice
Josué Gan is a PT working in a private practice in Bern, Switzerland. He is an instructor in trigger points therapy and dry needling for David G. Simons Academy. He is currently finishing his MSc in musculoskeletal at the University of Applied Sciences of Zurich, Switzerland.
Jacqueline Doidge has been a physical therapist since 1977, trained in Canada, and studied medical acupuncture and dry needling from 1987 to 1992 in South Africa. She lectured at University of Cape Town in manual therapies and trigger point techniques from 1987 to 1992. She has been in Arizona since 1994 and brought the ODNS dry-needling courses to USA in 2014. Since then, she has taught more than 35 courses in dry needling throughout the USA, Kenya, and the Middle East.
ORCID
Gary Kearns http://orcid.org/0000-0002-6530-2611 César Fernández-De-Las-Peñas http://orcid.org/00000003-3772-9690 Jean-Michel Brismée http://orcid.org/0000-0002-10377704 Josué Gan http://orcid.org/0000-0002-0058-2114 Jacqueline Doidge http://orcid.org/0000-0002-0498-4399
References
[1] Donnelly J, Fernández-de-Las-Peñas C, Finnegan M, et al. Myofascial pain and dysfunction: the trigger point manual. 3rd ed. Philadelphia (PA): Wolters Kluwer; 2018. [2] Travell JG, Simons DG. Myofascial pain and dysfunction: the trigger point manual; vol. 2., The lower extremities. 1st ed. Baltimore: LWW; 1992. [3] Kearns G, Gilbert KK, Allen B, et al. Accuracy and safety of dry needle placement in the piriformis muscle in cadavers. J Man Manip Ther. 2018;26(2):89-96. [4] Halle JS, Halle RJ. Pertinent dry needling considerations for minimizing adverse events – part one. Int J Sports Phys Ther. 2016;11(4):651-662. [5] Halle JS, Halle RJ. Pertinent dry needling considerations for minimizing adverse events – part two. Int J Sports Phys Ther. 2016;11(5):810-819. [6] Fernández-de-Las-Peñas C, Layton M, Dommerholt J. Dry Needling for the management of thoracic spine pain. J Man Manip Ther. 2015;23:3. [7] Brady S, McEvoy J, Dommerholt J, et al. Adverse events following trigger point dry needling: a prospective study of chartered physiotherapists. J Man Manip Ther. 2014;22:134-140. [8] Cummings M, Ross-Marrs R, Gerwin R. Pneumothorax complication of deep dry needling demonstration. Acupunct Med. 2014;32:517-519. [9] Dommerholt J, McEvoy J. Myofascial trigger point approach in orthopaedic manual physical therapy. In: Wise CH, editor. Orthopaedic manual physical therapy – from art to evidence. Philadelphia (PA): F.A. Davis Company; 2015. p. 378. [10] apta.org [Internet]. 2013. [cited Aug 23]. Available from: http://www.apta.org/StateIssues/DryNeedling/ ClinicalPracticeResourcePaper Alexandria (VA): The American Physical Therapy Association [11] fsbpt.org [Internet]. 2015. [cited Aug 23]. Available from: https://www.fsbpt.org/Portals/0/documents/ free-resources/DryNeedlingFinalReport_20150812.pdf Alexandria (VA): The Federation of State Boards of Physical Therapy [12] Dommerholt J, Fernández-de-Las-Peñas C, eds. Trigger point dry needling – an evidenced and clinical-based approach. Churchill Livingstone Elsevier; 2013. [13] Baldry P. Superficial dry needling. In: Dommerholt J, Fernández-de-Las-Peñas C, editors. Trigger point dry needling – an evidenced and clinical-based approach. Churchill Livingstone Elsevier; 2013:169-172. [14] Baldry P. Superficial versus deep dry needling. Acupunct Med. 2002;20(2-3):78-81. [15] Simons DG, Travel JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual; vol. 1. The upper half of body. 2nd ed. Baltimore: LWW; 1999. [16] Gunn CC. The gunn approach to the treatment of chronic pain: intramuscular stimulation for myofascial pain of radiculopathic origin. Vol. 2e. 2 ed. New York: Churchill Livingstone; 1996. [17] Hong C. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73:256-263. [18] Perreault T, Dunning J, Butts R. The local twitch response during trigger point dry needling: is it necessary for successful outcomes? J Bodyw Mov Ther. 2017;21:940-994. [19] Fernández-Carnero J, Gilarranz-de-Frutos L, León- Hernández JV, et al. Effectiveness of different deep dry needling dosages in the treatment of patients with cervical myofascial pain: a pilot RCT. Am J Phys Med Rehabil. 2017;96:726-733. [20] Taşoğlu Ö, Şahin Onat Ş, Bölük H, et al. Comparison of two different dry-needling techniques in the treatment of myofascial pain syndrome. Agri. 2017;29 (1):9-16. [21] McEvoy J. Trigger point dry needling: safety guidelines. In: Dommerholt J, Fernández-de-Las-Peñas C, editors. Trigger point dry needling – an evidenced and clinical-based approach. Churchill Livingstone Elsevier; 2013:39-56. [22] Wadell G, Newton M, Henderson I, et al. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157-168. [23] Dworkin RH, Turk DC, Trudeau JJ, et al. Validation of the short-form McGill Pain questionniare-2 (SF-MPQ- 2) in acute low back pain. J Pain. 2015;16(4):357-366. [24] Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizng scale: development and validation. Psychol Assess. 1995;7(4):524-532. [25] Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276-285. [26] Louw A, Nijs J, Puentedura E. A clinical perspective on pain neuroscience education approach to manual therapy. J Man Manip Ther. 2017;25:160-168. [27] Vas L, Phanse S, Pai R. New perspective of neuromyo- pathy to explain intractable pancreatic cancer pains; dry needling as an effective adjunct to neurolytic blocks. Indian J Palliat Care. 2016;22:85-93. [28] Shin HJ, Shin JC, Kim WS, et al. Application of ultra- sound-guided trigger point injection for myofascial trigger points in the subscapularis and pectoralis muscles to post- mastectomy patients: a pilot study. Yonsei Med J. 2014;55(3):792-799. [29] Fernández-Lao C, Cantarero-Villanueva I, Fernández- de-Las-Peñas C, et al. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled trial. Clin J Pain. 2012;28(2):113-121. [30] Goodman CC, Fuller KS. Pathology – implications for the physical therapist. 4th ed. Missouri (MO): Elsevier Saunders; 2015. [31] Goodman CC, Heick J, Lazaro RT. Differential diagnosis for physical therapists: screening for referral. 6th ed. Missouri (MO): Elsevier; 2018. [32] Berrigan WA, Whitehair C, Zorowitz R. Acute spinal epidural hematoma as a complication of dry needling: a case report. PM R. 2018 Jul 21. pii: S1934-1482 (18)30387-3. [33] Lee JH, Lee H, Jo DJ. An acute cervical epidural hematoma as a complication of dry needling. Spine (Phila Pa 1976). 2011;36:E891-3. [34] Osada A, Yamada H, Takehara S, et al. Gastrocnemius myalgia as a rare initial manifestation of CrohnLs dis- ease. Int Med. 2018 Feb 28. DOI:10.2169/internalme- dicine.0327-17. [35] Cristopoulos C, Savaas S, Pylarinou S, et al. Localised gastrocnemius myositis in CrohnLs disease. Clin Rheumatol. 2003;22(2):143-145. [36] Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95 (5):986-995. [37] Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018;99(2):383-389. [38] Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4-5):546- 549. [39] Wu ML, Yu KH, Tsai JC. The effectiveness of exercise in adults with systemic lupus erythematosus: a systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid Based Nurs. 2017;14(4):306- 315. [40] Haschka J, Englbrecht M, Heuber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic ther- apy: interim results from the prospective randomized controlled RETRO study. Ann Rheum Dis. 2016;75 (1):45-51. [41] Goodman SM, Bykerk VM, DiCarlo E, et al. Flares in patients with rheumatoid arthritis after total hip and total knee arthroplasty: rates, characteristics, and risk factors. J Rheumatol. 2018;45(5):604-611. [42] Chou PC, Chu HY. Clinical efficiency of Acupuncture on Rheumatoid arthritis and associated mechanisms: a systematic review. Evid Based Complement Alternat Med. 2018. DOI:10.1155/2018/8596918 8 G. KEARNS ET AL. [43] George SZ, Beneciuk JM, Bialosky JE, et al. Development of a review-of-systems screening tool for orthopaedic physical therapists: results from the optimal screening for prediction of referral and out- comes (OSPRO) Cohort. J Orthop Sports Phys Ther. 2015;45(7):512-526. [44] Payne WG, Naidu DK, Wheeler CK, et al. Wound Healing in Patients with Cancer. Eplasty. 2008 [Published online 2008 Jan 11];8:e9. [45] Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the peri- operative period. Am J Surg. 2013;206(3):410-417. [46] Hilton-Jones D. Statin-related myopathies. Pract Neurol. 2018;18(2):97-105. [47] Holder K. Myalgias and myopathies: drug-induced myalgias and myopathies. FP Essent. 2016;440:23-27. [48] Valiyil R, Christopher-Stine L. Drug-related myopathies of which the clinician should be aware. Curr Rheumatol Rep. 2010;12(3):213-220. [49] Boissonnault WG. Prevalence of comorbid conditions, surgeries, and medications use in a physical therapy outpatient population: a multicentered study. J Orthop Sports Phys Ther. 1999;29(9):506-519. [50] Coronado RA, Alappattu MJ, Hart DL, et al. Total number and severity of comorbidities do not differ based on anatomical region of musculoskeletal pain. J Orthop Sports Phys Ther. 2011;41(7):477-485. [51] Geerse WK. Bilateral leg symptoms – the T10 syndrome? Man Ther. 2012;17(3):251-254. [52] Sebastian D. Thoraco lumbar junction syndrome: a case report. Physiother Theory Pract. 2006;22(1):53-60. [53] Maigne R. Thoraco-lumbar junction syndrome: a source of diagnostic error. J Orthop Med. 1995;17 (3):84-89. [54] Cyriax J. Textbook of orthopaedic medicine. Volume one. Diagnosis of soft tissue lesions. Bailliere Tindall; 1982. [55] Boissonnault WG. Primary care for the physical therapist: examination and triage. 2nd ed. Missouri (MO): Elsevier Saunders; 2011. [56] Pastore EA, Katzman WB. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs. 2012;41:680-691. [57] Moldwin RM, Fariello JY. Myofascial trigger points of the pelvic floor: associations with urological pain syndromes and treatment strategies including injection therapy. Curr Urol Rep. 2013;14:409-417.