Effects of Adding a Neurodynamic Mobilization to Motor Control Training in Patients with Lumbar Radiculopathy due to Disc Herniation: A Randomized Clinical Trial. Plaza-Manzano G, et al. Am J Phys Med Rehabil. 2019 Sep 5. [Epub ahead of print]
Abstracted by:
Brent Denny, DPT, ScD, COMT Marble Falls, TX – Fellowship Candidate, IAOM-US Fellowship Program & Jean-Michel Brismée, PT, ScD, Fellowship Director, IAOM-US Fellowship program.
Context:
Contemporary evidence recommends conservative management as the first line treatment for low back pain (LBP) and radicular dysfunction. Evidence has demonstrated the effectiveness with movement based interventions, including motor control exercises (MCE) and various manual therapies. Neurodynamic mobilizations (NDM) have been demonstrated to improve intraneural fluid dispersion thereby reducing neural edema. Despite this the evidence supporting neurodynamic mobilization effectiveness in radicular pain is lacking.
Objective:
To determine the benefits of combining NDM with MCE in subjects suffering from lumbar radiculopathy.
Design:
Randomized Clinical Trial
Setting:
Clinical Setting
Participants:
Thirty-two people age 18 to 60 years with MRI confirmed L4-S1 disc herniations and radiating lower extremity pain for 3 to 5 months participated. Their pain had to be aggravated by cough, sneeze, strain and had provocative straight leg raise between 40-60 degrees hip flexion. Exclusion Criteria were absent reflexes, muscle atrophy, signs of lumbar myelopathy, disc herniations at other lumbar spinal levels, or had other spinal conditions.
Methods:
Participants were randomized into either the MCE (n=16) or combined MCE+NDM (n=16) groups. The treating therapist was blinded to subjects baseline examination results. Each group participated in 8 thirty-minute sessions twice weekly for 4 weeks and home based exercise program extended over an 8-week period. MCE included transverse abdominis and multifidus activation minimizing superficial muscle activity in various postures. Neurodynamic mobilization treatments included sciatic nerve “slider” techniques performed prior to MCE.
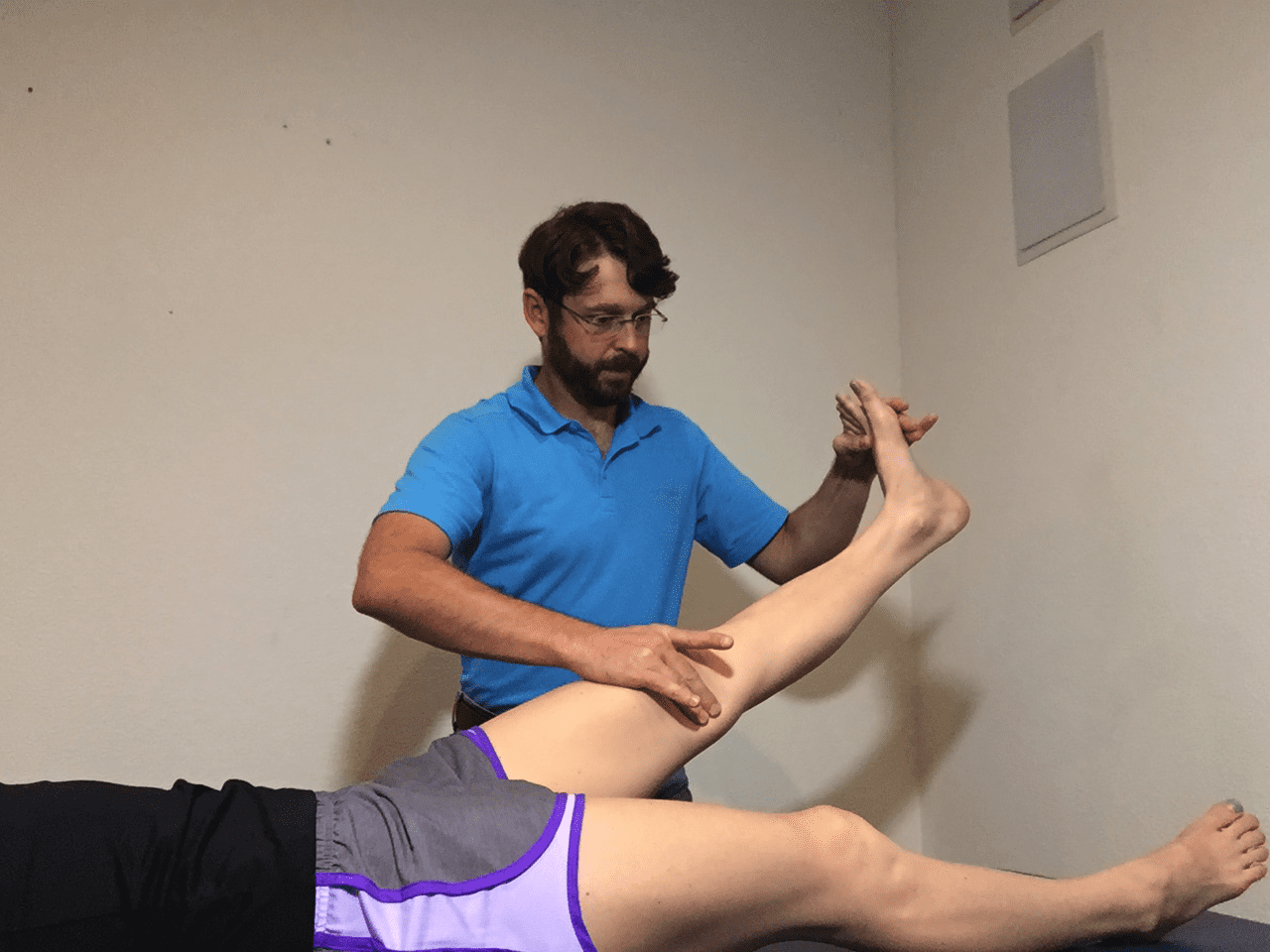
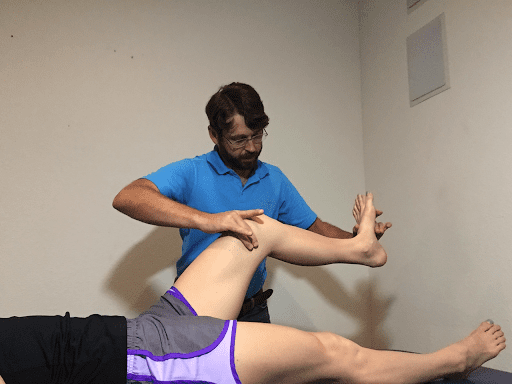

Figure 1: Sciatic neurodynamic mobilization: The knee is rhythmically extended and flexed while maintaining ankle dorsiflexion. Plaza-Manzano et al 2019
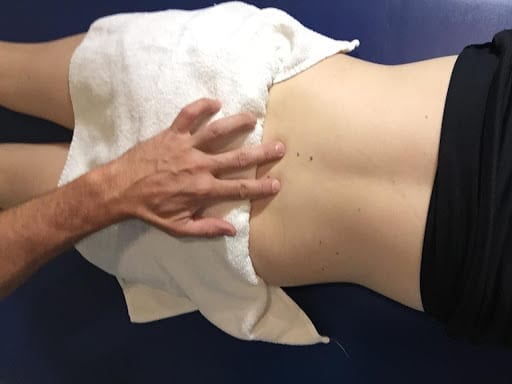


Figure 2: MCE Program: Isolated transverse abdominis & multifidus contraction progressed to combined training in supine, prone, bridge &
quadruped postures.
Outcome Measures:
| Measures | Method |
| Lower Extremity Pain | Numeric Pain Rating Scale: 0-10 (NPRS) |
| Sciatic Nerve Sensitivity | Passive Straight Leg Raise Test (SLR) |
| Neuropathic Symptoms | Self-Report Leeds Assessment of Neuropathic Symptoms (L-LANSS) |
| Disability | Roland-Morris Disability Questionnaire (RMDQ) |
| Pressure Pain Sensitivity | Pressure Pain Thresholds (PPT) of common peroneal nerve at fibular head and tibial nerve in popliteal fossa |
Results:
| Outcome Measure | Baseline | 2-Months | Between Group Effect Size | Within Group Effect Size | P-Value |
| NPRS (0-10) | |||||
| MCE | 6.0 +/-1.4 | 3.2 +/-0.8 | SMD: 0.2 (Small) | SMD >1.25 (Large) | P = 0.355 |
| MCE+NDM | 5.9 +/-1.4 | 2.6 +/- 0.8 | SMD >1.25 (Large) | ||
| SLR (o hip flexion ) | |||||
| MCE | 53.2 +/-10.0 | 63.1 +/- 12.8 | SMD: 0.55 (Moderate) – 1.05 (Large) | N/A | P = 0.013* |
| MCE+NDM | 55.2 +/- 6.5 | 71.9 +/- 9.8 | |||
| S-LANSS (0-24) | |||||
| MCE | 12.0 +/- 1.3 | 8.4 +/-1.5 | SMD: .95 – .75 (Large) | N/A | P = 0.008* |
| MCE+NDM | 12.0 +/- 1.1 | 6.5 +/- 1.6 | |||
| RMDQ (0-24) | |||||
| MCE | 10.5 +/-2.6 | 5.9 +/- 1.2 | SMD: 1.8 (Small) | SMD>1.15 (Large) | P = 0.101 |
| MCE+NDM | 11.2 +/- 1.5 | 5.2 +/- 1.4 | SMD>1.15 (Large) | ||
| PPT (kg/cm2) | |||||
| MCE | 2.3 +/- 1.0 | 2.8 +/- 0.8 | SMD: 0.14 (Small) | SMD>1.4 (Large) | P = 0.454 |
| MCE+NDM | 2.1 +/- 0.9 | 2.8 +/- 0.5 | SMD>1.4 (Large) | ||
Effect Size SMD: Standardized Mean Difference
95% confidence interval
Significant Difference: *
Conclusions:
The addition of NDM to MCE resulted in significant improvements in sciatic nerve sensitivity and neuropathic symptoms, however the effect size is small and the clinical relevance is questionable.
IAOM-US Commentary:
Plaza-Manzonoa et al (2019) demonstrated the utility that conservative, movement-based interventions have in LBP subjects with radicular, or nerve-root related symptoms. The addition of NDM to MCE did significantly improve sciatic nerve sensitivity and neuropathic symptoms, however, as the authors concluded based on the small effect size, the clinical relevance is questionable. Despite the small effect size NDM may still have a place in LBP with radicular treatments. Satpute et al 2019 concluded subjects with lumbar radiculopathy significantly improved in disability, pain, SLR and lumbar ROM in both short and long term when manual therapy was combined with NDM (See abstract by Kasey Miller PT, DPT, COMT in the IAOM-US academic commons https://iaom-us.com/the-effect-of-spinal-mobilization-with-leg-movement-in-patients-with-lumbar-radiculopathy-a-double-blind-randomized-controlled-trial/ ).
The IAOM-US proposes organization of treatment along the following spectrum. This will be referenced in the below commentary:
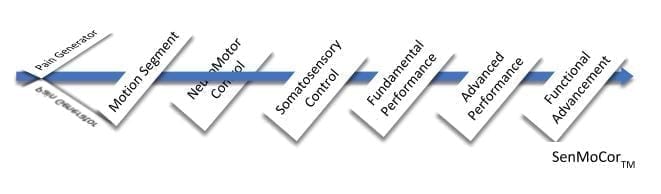
The lumbar spine is rich in tissues that are capable of producing a wide array of pain patterns. Reviewing lumbar pain patterns reveals how complex it is to define LBP and related dysfunctions. These pain-producing tissues are integral to lumbar spine movement. They should be taken into consideration when examining a person’s ability to move and may require specific pain-relieving treatment prior to sensorimotor training.
Different pain patterns arise and overlap depending on the anatomical structures involved. Structures innervated by the sinuvertebral nerve include the external fibers of the annular ligament (Konnai et al 2000), dura matter (Ohtori et al 1999), posterior longitudinal ligament, and anterior longitudinal ligament. The multi-segmental innervation of the sinuvertebral nerve leads to diffuse, non-radicular pain patterns that may refer to the low back, lower buttocks, leg, and groin. The inner annulus of the disc is mono-segmentally innervated and produces localized pain patterns (Sizer et al 2001). Neural tissue pain includes the nerve roots and the dorsal root ganglia. Both structures refer pain to the lower extremity and may be difficult to distinguish. Chemically mediated nerve root pain leads to gradual-onset aching sensations. The dorsal root ganglion, once chemically sensitized, produces an immediate increase in pain followed by after discharges that last for minutes in response to nociceptive mechanical stimulation (Sizer et al 2002). Root-related pain is a consequence of tension loading, secondary to acute disc disorders, or compression loading from age related segmental changes. The IAOM-US proposes dural tension testing using proximal and distal initiation to assess neural tissue response tension, proximal, and distal excursion. Sciatic nerve dural tension test interpretation is summarized in Table 1.
| Acute Disc Related Disorder | Recurrent Disc Related Disorder | Recurrent Disc Related Disorder | |
| Protrusion, Prolapse or Extrusion | Epidural Adhesions | Nerve Root Compression Syndrome or INC* | |
| Root Tension Event | Root Mobility Event | Root Compression Event | |
| Straight Leg Raise Distal Initiation (SLRDI) | |||
| Ankle DF + SLR | ↑ Pain | ↑↑ Pain* | ↑↑ Pain* |
| Chin Tuck + Neck Flexion | ↑↑ Pain* | ↓Pain | ↓Pain |
| Ankle PF | ↓Pain | – Pain | – Pain |
| Straight Leg Raise Proximal Initiation (SLRPI) | |||
| Chin Tuck + Neck Flexion | – Pain | – Pain | – Pain |
| Ankle DF + SLR | ↑↑ Pain* | – Pain | – Pain |
| Return Head to Mat | ↓ Pain | ↑↑ Pain* | ↑↑ Pain* |
| Slump Test Distal Initiation (SDI) | |||
| Ankle DF + Knee Ext | ↑↑ Pain | ↑↑ Pain* | – Pain |
| Neri + Trunk Slump | ↑↑↑ Pain* | ↓ Pain | – Pain |
| Ankle PF | ↓ Pain | – Pain | – Pain |
| Slump Test Proximal Initiation (SPI) | |||
| Neri + Trunk Slump | ↑ Pain | – Pain | – Pain |
| Ankle DF + Knee Ext | ↑↑↑ Pain* | – Pain | – Pain |
| Neck Extension+ Raise Head | ↓ Pain | ↑↑ Pain* | – Pain |
| DF: DorsiflexionPF: Plantarflexion
Neri: Chin Tuck + Neck Flexion Most Painful Test: * INC: Intermittent Neurogenic Claudication |
|||
| Table 1: Sciatic nerve dural tension testing interpretation adapted from Sizer et al 2002. | |||
Straight leg raise distal initiation is represented in figure 3 and proximal initiation in figure 4. Slump distal and proximal initiations are both represented in figure 5.
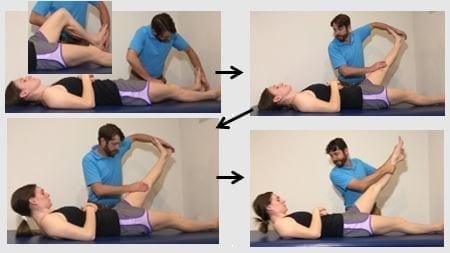
Figure 3: Straight leg raise distal initiation: Key features: if tolerated the test should not be performed with a pillow under the head. The knee is first flexed prior to ankle dorsiflexion to provide maximal distal neural tension. Maintain full available knee extension.
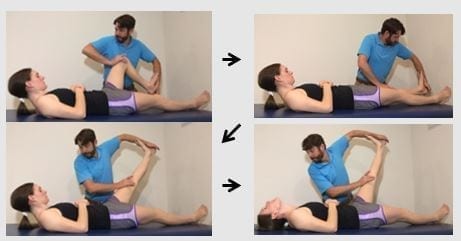
Figure 4: Straight leg raise proximal initiation: Key features: if tolerated do not place a pillow under the head. The knee is first flexed prior to cervical + head flexion to provide maximal proximal neural tension. Maintain full available knee extension.
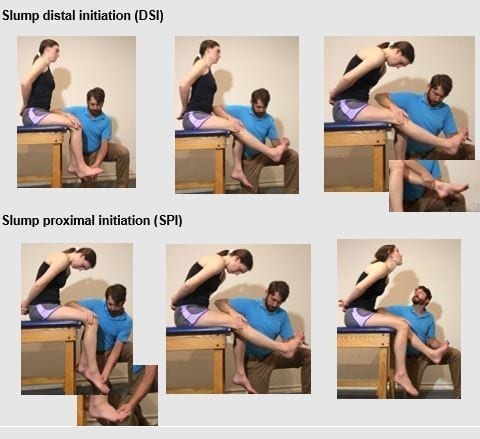
Figure 5: Slump Testing: Key feature: Position client so they are sitting on their ischial tuberosity’s. Do not allow the client to roll onto their sacrum as you increase dural tension.
Prior to initiation of NDM for root-related pain the IAOM-US recommends performing targeted treatments directed at the underlying cause. For instance, with acute disc related disorders 3-dimensional axial separation, directional preference postures and/or movements, and traction may be used to reduce the tension loading on the involved dura. Client positioning may be supine (Figure 6) or in prone on elbows if they have an extension directional preference (Figure 7). Neurodynamic mobilizations may be used to prevent epidural scarring once the tension loading has reduced. In nerve root compression syndrome and INC intermittent traction may be employed to “rinse” the venous plexus of Baston followed by NDM (Sizer et al 2002). Stenotic clients may respond with NDM performed in sitting with slight contralateral side bending to open the foramen (Figure 7). Moreover, the time of day influences decisions regarding when to use NDM. Acute disc clients tend to have higher pain levels in the morning when the disc is hydrated (Snook et al 1998). It may be prudent to perform NDM in the afternoon or evenings after the discs are dehydrated resulting in less dural tension loading. Recurrent disc related clients often complain of higher pain levels in the evenings when the disc is dehydrated. They may benefit from NDM in the morning when plumper disc contribute to less segmental narrowing and dural tissue compression loading. The clinician is cautioned to initiate NDM conservatively with the following quote:
“You are essentially tickling the dragon’s tail and there is a chance that you could get burned and the symptoms worsen. Flossing can both cure and cause pain.”
- Dr. Stuart McGill
Online Course: Neural Mobilization Concepts
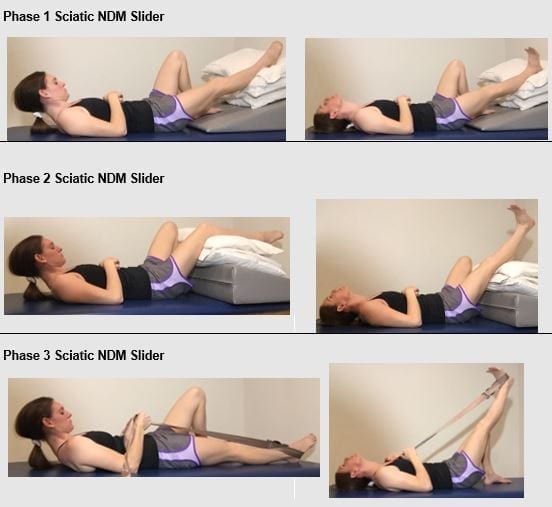
Figure 6: IAOM-US proposed supine sciatic NDM progression. Note the hip is slightly abducted and externally rotated to avoid tension loading the sciatic nerve around the ischial tuberosity. Further progression may be achieved with trunk flexion (not pictured).
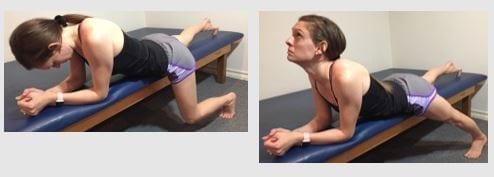
Figure 7: Sciatic NDM “slider” in prone on elbows recommended for clients with an extension directional preference.

Figure 8: Seated sciatic NDM with left lateral flexion for right nerve root compression syndrome.
The Motor Control Exercises used by Plaza-Manzonoa et al (2019) focused on transverse abdominis (TrA) and multifidus (Mf) training in various postures. Multiple studies demonstrate a deficit in trunk muscle activation timing in painful subjects (Akbari et al 2015, Kiesel et al 2008, MacDonald et al 2010, Leinonen et al., 2001, Goubert et al 2016) . There appears to be consistent local spinal muscle hypoactivity and a global muscle hyperactivity in the presence of, as well as a history of, low back pain; a manifestation of control alterations. The TrA feedforward contraction is often delayed in people with a LBP history compared to non-painful controls (Hodges et al 1996, Hodges et al 1998, Hodges et al 2003). This represents diminished feedforward, preparatory activity. The lumbar Mf is susceptible to maladaptive changes in the LBP population. Histochemical changes involve diminished cross-sectional area of Type I (slow-twitch tonic) and Type II (fast-twist phasic) fibers, reduced percentage of Type II fibers, and increased interstitial fibrosis (Zhao et al 2000). Compared to non-painful subjects, magnetic resonance images of the Mf reveal significantly more muscle atrophy in LBP subjects. Mild atrophy is described as less than 10% replacement of muscle bulk with fatty and fibrous tissue while severe atrophy is greater than 50% replacement (Kader et al 2000). Multifidus atrophy and diminished activation persist beyond the painful time frame. Compared to subjects without a LBP history, recurrent LBP subjects continue to display significantly slower onset of the feedforward deep Mf activation compared to the superficial Mf during anticipated extremity movement. The activation delay was reported to be exaggerated ipsilateral to the side that has been previously painful (MacDonald et al 2010). Somatosensory deficits resulting from current LBP, or a history of LBP, include loss of proprioception (Newcomer et al 2000, Leinonen et al., 2002, O’sullivan et al., 2003, O’Sullivan et al., 2013, Tong et al., 2017), feed-back based, reactive activation (Radebold et al 2000, Radebold et al 2001), postural control (Mok et al 2004, Mok et al 2013), and balance (Hooper et al 2016).
Volitional, pre-emptive abdominal controcation (VPAC) strategies focus on functional trunk muscle activation (Nagar et al 2014). The VPAC comprises strategies to target local muscles including the TrA, Mf, and internal obliques (IO) using the abdominal drawing in maneuver (ADIM). Global muscles including the external oblique (EO), erector spinae (ES), rectus abdominis (RA) are activated using the abdominal brace manevere (ABM). Given the propensity for global hyper-activity, early feed forward reactivation may focus the ADIM reserving the ABM for more demanding activities further down the treatement spectrum.
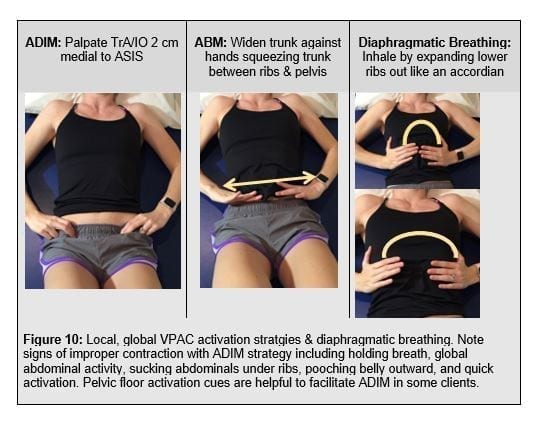
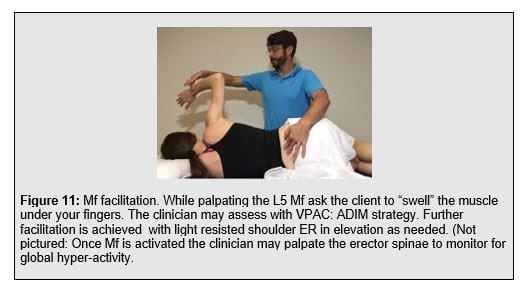
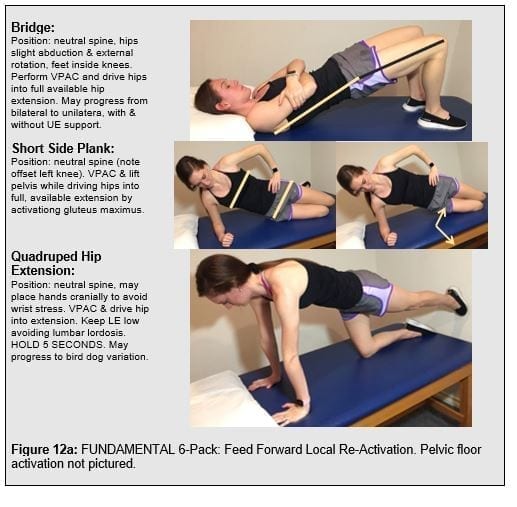
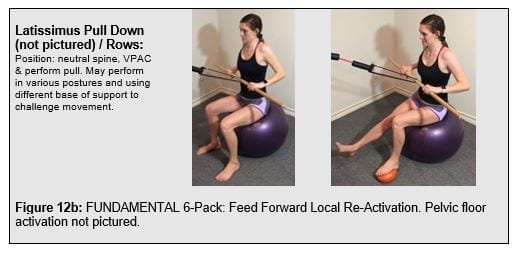
Expanding the local trunk locomotor re-activation beyond the TrA/IO, Mf to include pelvic floor muscles (PFM), gluteals, and latissimus dorsi to pair the volitional trunk stiffness with the functioal posterior oblique sling. This comprises the “functional 6-pack” discussed in the SenMoCor courses offered by IAOM-US (Figure 12).
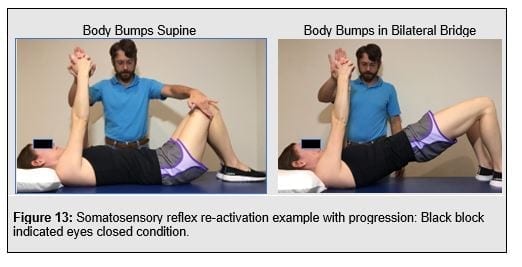
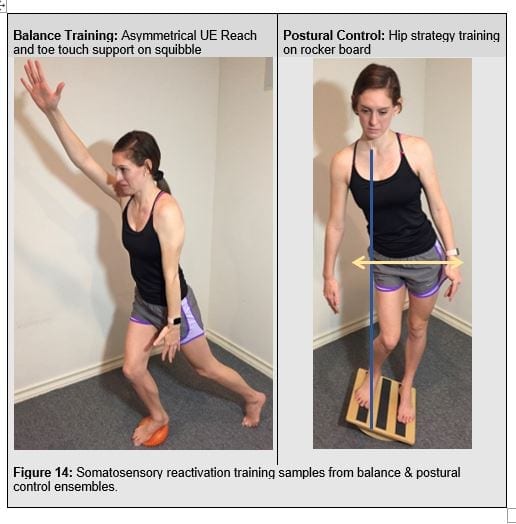
Somatosensory re-activation, or reactive sensoirmotor control, involves reflex re-activation training (Figure 13) whereby multi-modale, unexpected perturbations should be employed Postural stability and control requires re-training hip strategy into their postural repertoire. Balance re-activation training focuses on the ability to control the center of mass within the base of support (figure 14).
Both locomotor and somatosensory re-activation may be treated concurrently with tissue and joint specific techniques used to address the pain generator and motion segment dysfunctions. As sufficient progress is achieved the IAOM-US recommends shifting treatment focus away from specific tissue dysfunction towards specific movement dyfuntion. Indicative with this shift is the change from specific musle re-activation to improving movement patterns.
To examine, interpret, and quantify movement-based dysfunction the lower quarter functional examination is proposed. This examination assesses direction specific movement dysfunctions (i.e. extension and/or rotation control deficits), and how their system responds with VPAC in the context of progressing developmental postures. Based on this information specific corrective strategy progressions address specific movement control deficits. Advanced performance and functional advancement follow corrective strategeies in the form of composite movements and exercises that are further progressed with speed and loading towards the clients functional needs.
References
- Akbari M, Sarrafzadeh J, Maroufi N, Haghani H. Changes in postural and trunk muscles responses in patients with chronic nonspecific low back pain during sudden upper limb loading. Med J Islam Repub Iran. 2015;29:265.
- Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural Changes of Lumbar Muscles in Non-Specific Low Back Pain. Pain Physician. 2016;19:E985-E1000.
- Hodges PW, Richardson CA. Inefficient Muscular Stabilization of the Lumbar Spine Associated With Low Back Pain: A Motor Control Evaluation of Transverse Abdominis. SPINE. 1996;21(22):2640-2650.
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77(2):132–142
- Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13(4):361-370.
- Hooper TL, James CR, Brismée J-M, et al. Dynamic balance as measured by the Y-Balance Test is reduced in individuals with low back pain: A cross-sectional comparative study. Phys Ther Sport. 2016;22:29-34
- Kiesel KB, Uhl T, Underwood FB, Nitz AJ. Rehabilitative ultrasound measurement of select trunk muscle activation during induced pain. Man Ther. 2008;13(2):132-138.
- Kader DF, Wardlaw D, Smith FW. Correlation Between the MRI Changes in the Lumbar Multifidus Muscles and Leg Pain. Clin Radiol. 2000;55(2):145-149
- Leinonen V, Kankaanpää M, Luukkonen M, et al. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine. 2003;28(8):842–848
- MacDonald D, Moseley GL, Hodges PW. People with recurrent low back pain respond differently to trunk loading despite remission from symptoms. Spine. 2010;35(7):818–824.
- Mok NW, Brauer SG, Hodges PW. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine. 2004;29(6):E107–E112
- Mok NW, Hodges PW. Movement of the lumbar spine is critical for maintenance of postural recovery following support surface perturbation. Exp Brain Res. 2014;231(3):305-313
- Nagar VR, Hooper TL, Dedrick GS, Brismée J-M, Sizer PS. Effect of Recurrent Low Back Pain History on Volitional Pre-emptive Abdominal Activation During a Loaded Functional Reach Activity: Spine. 2014;39(2):E89-E96
- Newcomer KL, Laskowski ER, Yu B, Johnson JC, An K-N. Differences in repositioning error among patients with low back pain compared with control subjects. Spine. 2000;25(19):2488–2493
- McGill, S. Back Mechanic: The secrets to a healthy spine your doctor isn’t telling you.1st Gravenhurst, Ontario, Canada;2015.
- Ohtori S, Takahashi Y, Takahashi K, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24(22):2295.
- O’Sullivan K, Verschueren S, Van Hoof W, Ertanir F, Martens L, Dankaerts W. Lumbar repositioning error in sitting: Healthy controls versus people with sitting-related non-specific chronic low back pain (flexion pattern). Man Ther. 2013;18(6):526-532.
- O’sullivan PB, Burnett A, Floyd AN, et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28(10):1074–1079.
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–954
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724–730
- Satpute, K, Hall T, Bisen R, Lokhande, P. The Effect of Spinal Mobilization With Leg Movement With Leg Movement in Patients with Lumbar Radiculopathy-A Double-Blind Randomized Controlled Trial. Arch Phys Med & Rehab. 2019;100(5): 829-836.
- Sizer Jr PS, Matthijs O, Phelps V. Influence of Age on the Development of Pathology. Curr Rev Pain. 2001;4(5):362-73.
- Sizer PS, Phelps V, Dedrick G, Matthijs O. Differential Diagnosis and Management of Spinal Nerve Root-related Pain. Pain Pract. 2002;2(2):98–121.
- Snook SS. The reduction of chronic nonspecific low back pain through the control of early morning lumbar flexion: a randomized controlled trial. 1998;23:2601–2607.
- Zhao W-P, Kawaguchi Y, Matsui H, Kanamori M, Kimura T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine. 2000;25(17):2191–2199.
